Abstract
Development of inhibitors to Factor VIII is a serious and not uncommon (occurs in about 30% of treated patients) complication of the treatment with factor concentrate in hemophilia A. Treatment of patients with inhibitors is complicated, expensive and not always successful. By-pass agents (rFVIIa and aPCC) have been considered as a treatment of choice for those patients. Very recently bispecific factor IXa- and factor X-directed antibody - Emicizumab has been approved for the treatment of hemophilia A patients with inhibitors. However, there are data from the clinical studies which raise concerns about potential prothrombotic effect of Emicizumab in combination with by-pass agents particularly aPCC.
To elucidate a potential mechanism of hypercoagulability we have investigated fibrin clot quality in hemophilic plasma after addition of a sequence identical analogue (SIA) of Emicizumab in combination with by-pass agents.
Parameters of fibrin clot turbidity (lag time, Max Abs, Slope, Slope time) were measured in recalcified FVIII-deficient plasma samples, after addition of low thrombin concentration (0.04 NIH/mL) and different concentrations of SIA (200 and 600 nM), rFVIIa (1.75 and 5.25 µg/mL) and aPCC (50 and 500 mU/mL). Pooled normal plasma (PNP) was used as control.
After fixation, fibrin clots were analyzed by scanning electron microscopy (SEM) (Carl Zeiss, Oberkochen, Germany) and the thickness of individual fibers was measured using SIS iTEM software (FEI Company, Eindhoven, Netherlands). As previously determined, 50 randomly selected fibers in each sample were measured to obtain stable mean.
All parameters of fibrin turbidity and structure were analyzed in triplicate and the results are presented as mean values.
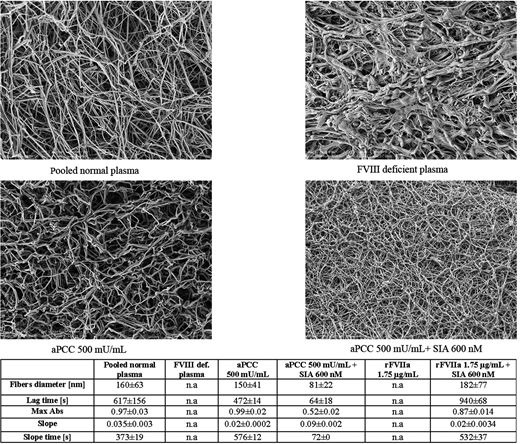
Fibrin clot in FVIII deficient plasma was difficult to analyze due to the lack of polymerization of fibrin monomers and absence of fiber formation. This structure is loose and prone to fibrinolysis. Interestingly the similar structure was observed after addition of both concentrations of rFVIIa while after addition of SIA fibrin structure improves. The best effect using single agent was noticed after addition of aPCC in concentration of 500 mU/mL. In combination with SIA (both concentrations of 200 nM and 600 nM) aPCC in concentrations of 500 mU/mL generated clots with highest density formed from very thin fibers and small intrinsic pores. These clots are even tighter than those formed from PNP samples.
Parameters of fibrin turbidity obtained with higher concentrations of agents as well as representative SEM scans are presented on the Figure 1.
Addition of SIA to by-pass agents additionally improved fibrin quality in FVIII deficient plasma. Combination of therapeutic concentrations of aPCC and SIA may produce clots even less porous than those in normal plasma.
Chaireti:Shire: Research Funding. Antovic:NovoNordisk: Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Werfen: Honoraria; Stago: Honoraria; Siemens: Honoraria; Roche: Honoraria; Sysmex: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal